Condensation polymer

Condensation polymers are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as byproducts such as water or methanol. Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization, when the polymer is formed by sequential addition (by condensation reaction) of monomers to an active site in a chain reaction. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers.
A polymerization in which the growth of polymer chains proceeds by condensation reactions between molecules of all degrees of polymerization. Notes: 1. The growth steps are expressed by:
Px+Py→Px+y+L {x}∈{1,2,…∞};{y}∈{1,2,…∞} where Px and Py denote chains of degrees of polymerization x and y, respectively, and L a low-molar-mass by-product.
2. The earlier term 'polycondensation' was synonymous with 'condensation polymerization'. The current definitions of polycondensation and condensative chain polymerization were both embraced by the earlier term 'polycondensation'.[1]Condensation polymerization is a form of step-growth polymerization. Linear polymers are produced from bifunctional monomers, i.e. compounds with two reactive end groups. Common condensation polymers include polyamides, polyacetals, and proteins.[2][3]
Polyamides[edit]
One important class of condensation polymers are polyamides.[4] They arise from the reaction of carboxylic acid and an amine. Examples include nylons and proteins. When prepared from amino-carboxylic acids, e.g. amino acids, the stoichiometry of the polymerization includes co-formation of water:
- n H2N-X-CO2H → [HN-X-C(O)]n + n H2O
When prepared from diamines and dicarboxylic acids, e.g. the production of nylon 66, the polymerization produces two molecules of water per repeat unit:
- n H2N-X-NH2 + n HO2C-Y-CO2H → [HN-X-NHC(O)-Y-C(O)]n + 2n H2O
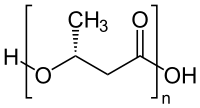
Polyesters[edit]
One important class of condensation polymers are polyesters.[5] They arise from the reaction of carboxylic acid and an alcohol. Examples include polyesters, e.g. polyethyleneterephthalate:
- n HO-X-OH + n HO2C-Y-CO2H → [O-X-O2C-Y-C(O)]n + (3n-2) H2O
Safety and environmental considerations[edit]
Condensation polymers tend to be more biodegradable than addition polymers. The peptide or ester bonds between monomers can be hydrolysed, especially in the presence of catalysts or bacterial enzymes.[citation needed]
See also[edit]
References[edit]
- ^ Jenkins, A. D.; Kratochvíl, P.; Stepto, R. F. T.; Suter, U. W. (1996-01-01). "Glossary of basic terms in polymer science (IUPAC Recommendations 1996)". Pure and Applied Chemistry. 68 (12): 2287–2311. doi:10.1351/pac199668122287. ISSN 0033-4545.
- ^ Introduction to Polymers 1987 R.J. Young Chapman & Hall ISBN 0-412-22170-5
- ^ D. Margerison, G. C. East, J. E. Spice (1967). An Introduction to Polymer Chemistry. Pergamon Press. ISBN 978-0-08-011891-8.CS1 maint: uses authors parameter (link)
- ^ B. Herzog, M. I. Kohan, S. A. Mestemacher, R. U. Pagilagan, K. Redmond (2013). "Polyamides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_179.pub3.CS1 maint: uses authors parameter (link)
- ^ Horst Köpnick, Manfred Schmidt, Wilhelm Brügging, Jörn Rüter, Walter Kaminsky (2002). "Polyesters". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_227.CS1 maint: uses authors parameter (link)
External links[edit]
| Wikimedia Commons has media related to Condensation polymerization. |

No comments:
Post a Comment